Feb 7, 2024 | Main Page Post, Rejuvenair, SPRAY-CB
The RejuvenAir System is a minimally invasive medical device therapy which aims to target the underlying cause of COPD with Chronic Bronchitis BOSTON, Feb. 7, 2024 /PRNewswire/ — CSA Medical Inc., a developer of medical devices advancing the power of liquid...
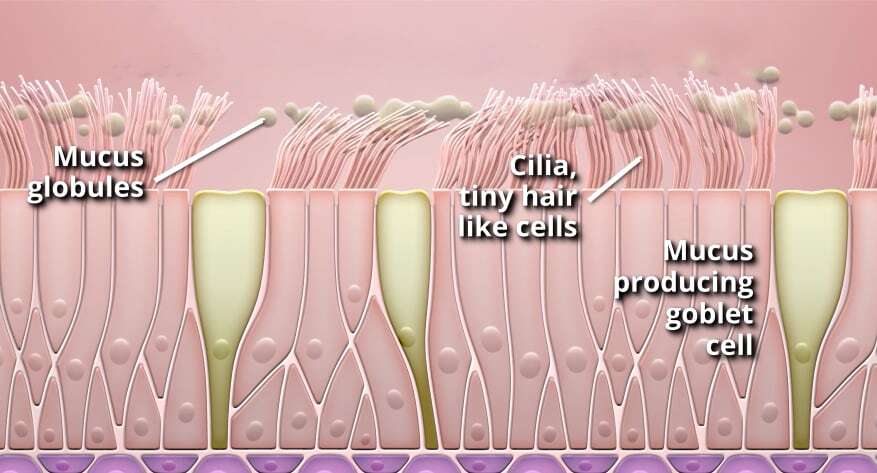
May 13, 2021 | Main Page Post, Product News, Rejuvenair, SPRAY-CB
These results should provide the scientific proof behind the remodeling response and clinical improvements identified in earlier studies Boston, MA—May 12, 2021: CSA Medical, Inc., today announced ® that all subjects have been enrolled in the RejuvenAir System study...

Apr 19, 2021 | Main Page Post, Product News, Rejuvenair, SPRAY-CB
Boston, MA –April 19, 2021: CSA Medical, Inc., today announced that Wissam Abouzgheib, MD, chief of pulmonary medicine at Cooper University Health Care, Camden, New Jersey, has joined the SPRAY-CB Study investigating a new device treatment for COPD with Chronic...

Oct 22, 2020 | Main Page Post, SPRAY-CB
The SPRAY-CB trial marks the first pivotal study of a medical device therapy targeting the underlying cause of Chronic Bronchitis. Boston,MA. – October 22, 2020: CSA Medical Inc., a developer of medical devices advancing the power of liquid nitrogen spray cryotherapy,...

Dec 4, 2019 | Main Page Post, Rejuvenair, SPRAY-CB
Published by Mass Device Medical Network CSA Medical announced this week that it won CE Mark approval for its RejuvenAir system for treating chronic obstructive pulmonary disease (COPD) with chronic bronchitis (CB). The RejuvenAir system that is designed to apply...




